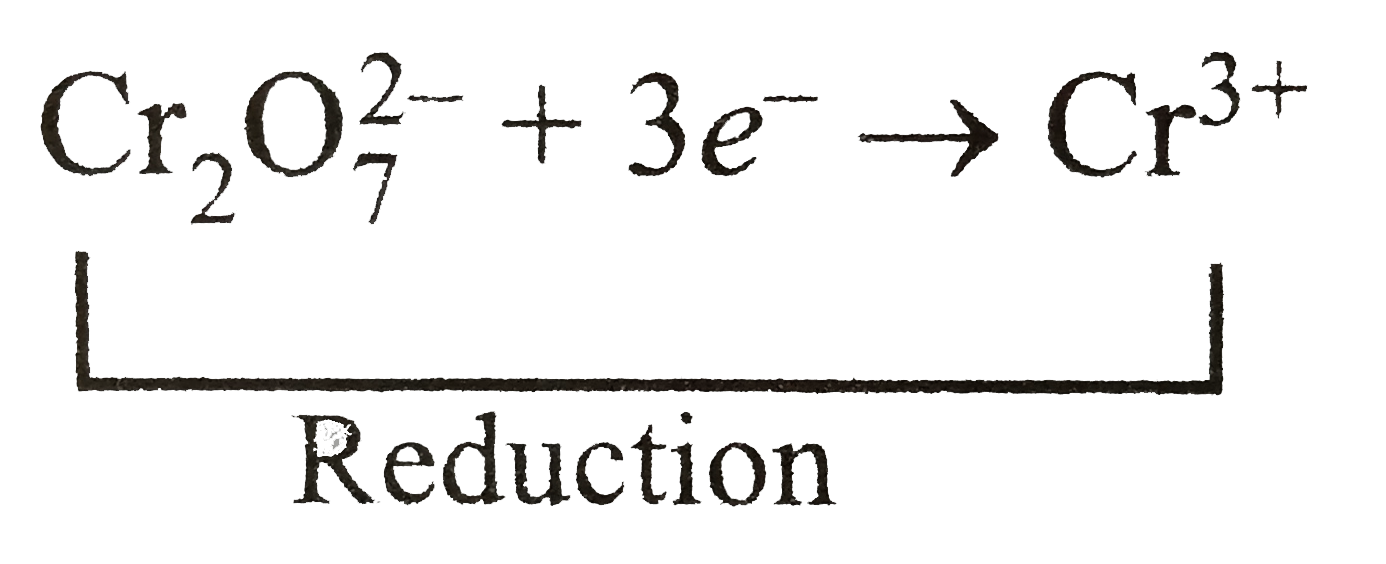SOLVED: Balance the following reactions in an acidic solution (not all will need Ht ions) Cr (s) + Ni2+ 7 Cr 3+ (aq) + Ni (s) 2. Clz (aq) + Br 7

Investigating CO2 Sorption in SIFSIX-3-M (M = Fe, Co, Ni, Cu, Zn) through Computational Studies | Crystal Growth & Design
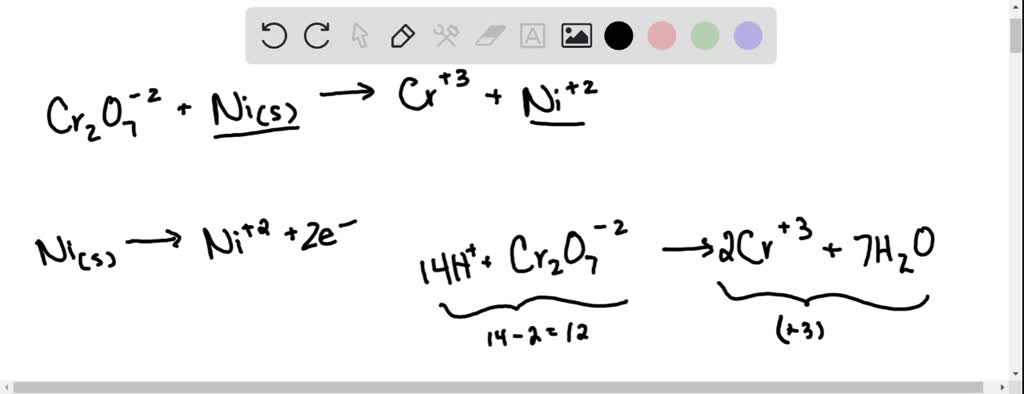
SOLVED: Balance the following redox equation in an acidic solution : Cr2O7 -2 (aq) + Ni (s) —– > Ni2+ (aq) + Cr3+ (aq) (acidic solution)

Calculate the equilibrium constant for the reaction: 3Sn(s) + 2Cr2O7^2 - + 28 H^+→ 3 Sn^+4 + 4Cr^3 + + 14H2O E^∘ for Sn/Sn^2 + = 0.136 V E^∘ for Sn^2 + /
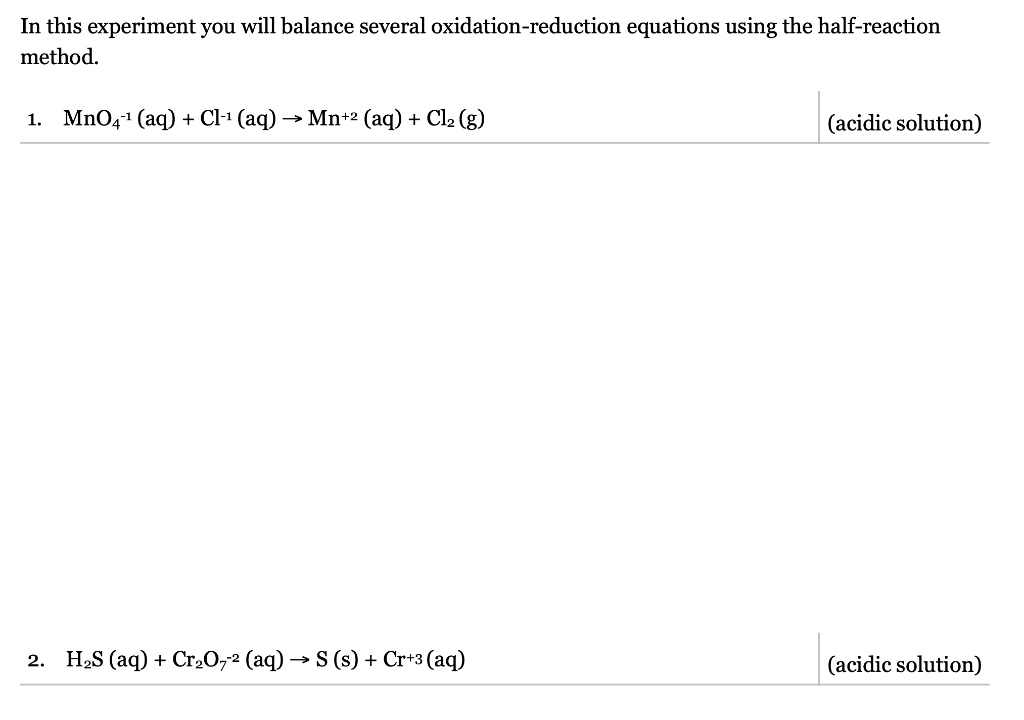
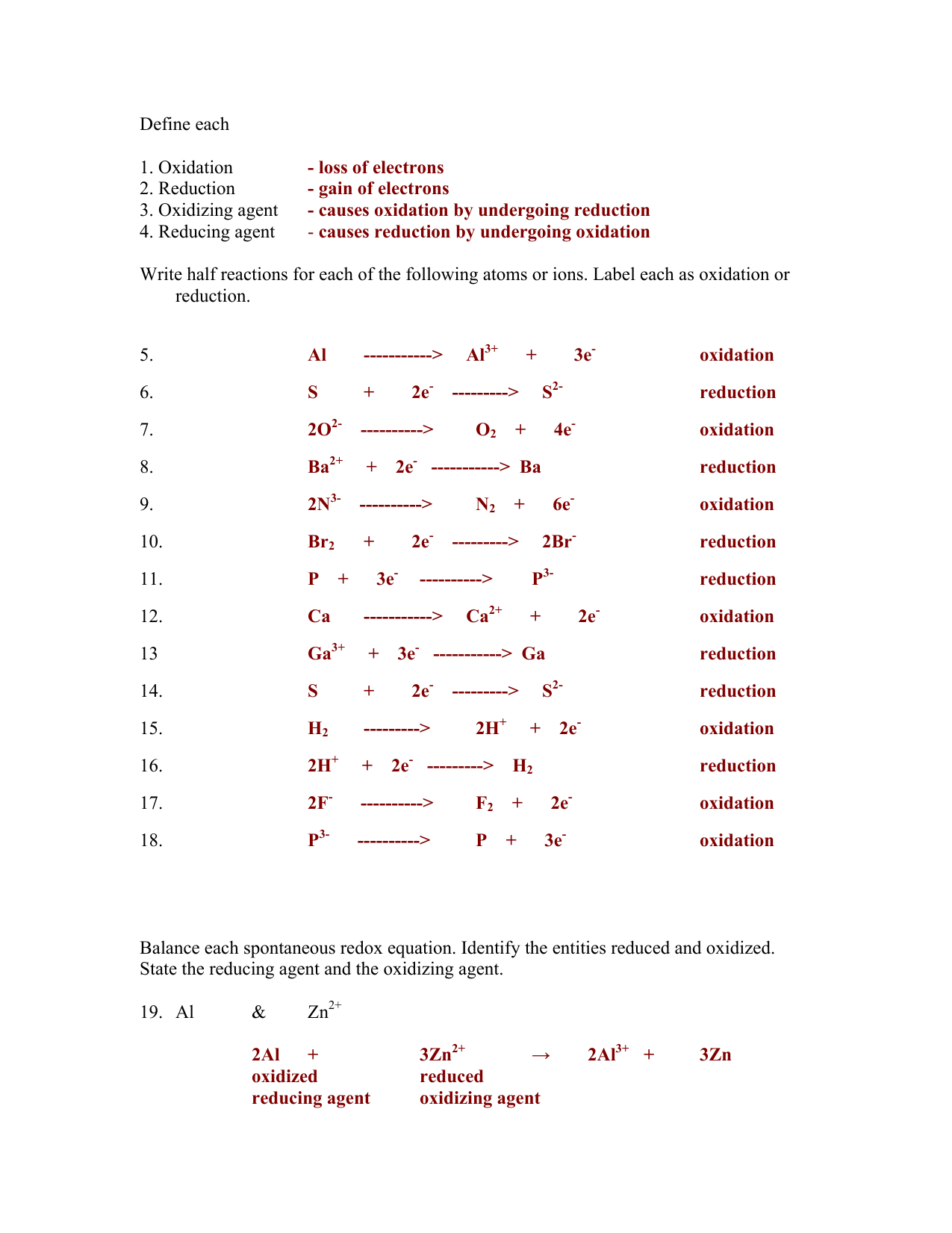
SOLVED: In this experiment you will balance several oxidation-reduction equations using the half-reaction method. 1.MnO4-1 (aq) + Cl-1 (aq) -> Mn+2 (aq) + Cl2 (g) (acidic solution) 2.H2S (aq)+ Cr2O7-2 (aq) ->

IB chemistry online - Transition elements-Colors: From left to right: Ti+2, V+3, VO2+, Cr+3, Cr2O7-2, Mn+2, MnO4-1, Fe+3, Co+2, Ni+2, Cu+2 | Facebook

Write the name and formula of the chemical reagent used for the diagnosis of Ni^2+ ion in a solution. | Homework.Study.com

Chromium transition metal Chemistry chromium(III) Cr3+ complex ions chromate(VI) CrO42- dichromate(VI)Cr2O72- redox chemical reactions principal +3 +6 oxidation states ligand substitution GCE AS A2 IB A level inorganic chemistry revision notes